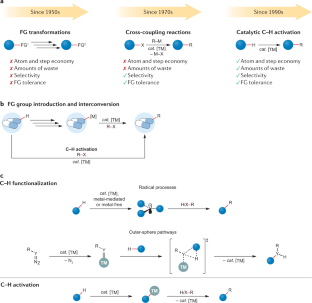
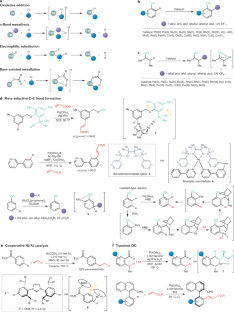
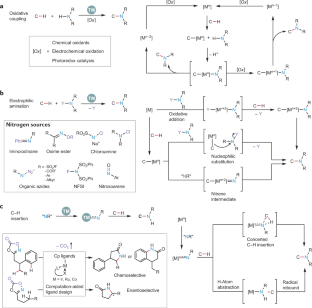
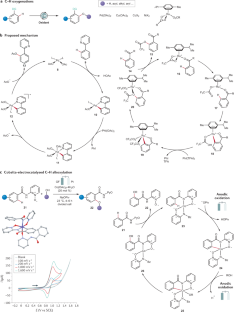
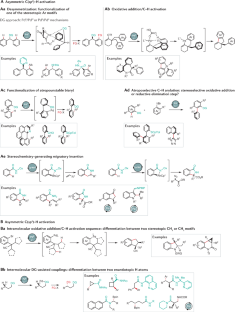
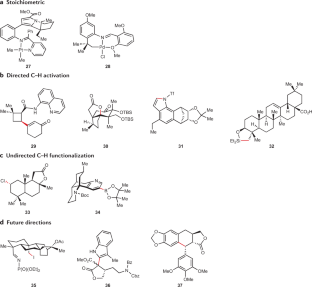
C–H activation
Transition metal-catalysed C–H activation has emerged as an increasingly powerful platform for molecular syntheses, enabling applications to natural product syntheses, late-stage modification, pharmaceutical industries and material sciences, among others. This Primer summarizes representative best practices for the experimental set-up and data deposition for C–H activation, as well as discussing key developments including recent advances in asymmetric, photoinduced and electrocatalytic C–H activation. Likewise, strategies for applications of C–H activation towards the assembly of structurally complex (bio)polymers and drugs in academia and industry are discussed. In addition, current limitations in C–H activation and possible approaches for overcoming these shortcomings are reviewed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
133,45 € per year
only 133,45 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Transient directing ligands for selective metal-catalysed C–H activation
Article 20 July 2021
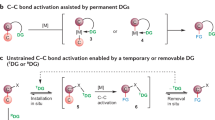
Temporary or removable directing groups enable activation of unstrained C–C bonds
Article 21 September 2020
Transition metal-catalysed directed C–H functionalization with nucleophiles
Article 27 October 2022
References
- Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed.51, 5062–5085 (2012). Google Scholar
- Nicolaou, K. C., Bulger, P. G. & Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed.44, 4442–4489 (2005). Google Scholar
- Khake, S. M. & Chatani, N. Chelation-assisted nickel-catalyzed C–H functionalizations. Trends Chem.1, 524–539 (2019). Google Scholar
- Gandeepan, P. et al. 3d transition metals for C–H activation. Chem. Rev.119, 2192–2452 (2019). Google Scholar
- Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev.47, 6603–6743 (2018). Google Scholar
- Wang, C. S., Dixneuf, P. H. & Soule, J. F. Photoredox catalysis for building C–C bonds from C(sp 2 )–H bonds. Chem. Rev.118, 7532–7585 (2018). Google Scholar
- Chu, J. C. K. & Rovis, T. Complementary strategies for directed C(sp 3 )–H functionalization: a comparison of transition-metal-catalyzed activation, hydrogen atom transfer, and carbene/nitrene transfer. Angew. Chem. Int. Ed.57, 62–101 (2018). Google Scholar
- Murakami, K., Yamada, S., Kaneda, T. & Itami, K. C–H functionalization of azines. Chem. Rev.117, 9302–9332 (2017). Google Scholar
- Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev.117, 9247–9301 (2017). Google Scholar
- He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev.117, 8754–8786 (2017). Google Scholar
- Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci.2, 281–292 (2016). Google Scholar
- Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature417, 507–514 (2002). ADSGoogle Scholar
- Egorova, K. S. & Ananikov, V. P. Toxicity of metal compounds: knowledge and myths. Organometallics36, 4071–4090 (2017). Google Scholar
- Allian, A. D. et al. Process safety in the pharmaceutical industry—part I: thermal and reaction hazard evaluation processes and techniques. Org. Process. Res. Dev.24, 2529–2548 (2020). Google Scholar
- Baumann, M., Moody, T. S., Smyth, M. & Wharry, S. A perspective on continuous flow chemistry in the pharmaceutical industry. Org. Process. Res. Dev.24, 1802–1813 (2020). Google Scholar
- Meyer, T. H., Finger, L. H., Gandeepan, P. & Ackermann, L. Resource economy by metallaelectrocatalysis: merging electrochemistry and C–H activation. Trends Chem.1, 63–76 (2019). Google Scholar
- Chatt, J. & Davidson, J. M. 154. The tautomerism of arene and ditertiary phosphine complexes of ruthenium(0), and the preparation of new types of hydrido-complexes of ruthenium(II). J. Chem. Soc.https://doi.org/10.1039/JR9650000843 (1965). ArticleGoogle Scholar
- Cope, A. C. & Siekman, R. W. Formation of covalent bonds from platinum or palladium to carbon by direct substitution. J. Am. Chem. Soc.87, 3272–3273 (1965). Google Scholar
- Janowicz, A. H. & Bergman, R. G. Carbon–hydrogen activation in completely saturated hydrocarbons: direct observation of M + R–H → M(R)(H). J. Am. Chem. Soc.104, 352–354 (1982). This investigation represents a pioneering study on the activation of unactivated C–H bonds via oxidative addition to a coordinatively unsaturated, in situ-generated iridium(I) complex. Google Scholar
- Kleiman, J. P. & Dubeck, M. The preparation of cyclopentadienyl [o-(phenylazo)phenyl]nickel. J. Am. Chem. Soc.85, 1544–1545 (1963). Google Scholar
- Omae, I. Intramolecular five-membered ring compounds and their applications. Coord. Chem. Rev.248, 995–1023 (2004). Google Scholar
- Duff, J. M. & Shaw, B. L. Complexes of iridium(III) and rhodium(III) with metallated and unmetallated dimethyl(1-naphthyl)- and methylphenyl(1-naphthyl)-phosphine. J. Chem. Soc. Dalton Trans.https://doi.org/10.1039/DT9720002219 (1972). ArticleGoogle Scholar
- De Sarkar, S., Liu, W., Kozhushkov, S. I. & Ackermann, L. Weakly coordinating directing groups for ruthenium(II)-catalyzed C–H activation. Adv. Synth. Catal.356, 1461–1479 (2014). Google Scholar
- Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res.45, 788–802 (2012). Google Scholar
- Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev.111, 1315–1345 (2011). Google Scholar
- Balcells, D., Clot, E. & Eisenstein, O. C–H bond activation in transition metal species from a computational perspective. Chem. Rev.110, 749–823 (2010). This review analyses various mechanistic manifolds of transition metal-catalysed C–H activation in detail and classifies these based on computational investigations. Google Scholar
- Ackermann, L., Vicente, R. & Althammer, A. Assisted ruthenium-catalyzed C–H bond activation: carboxylic acids as cocatalysts for generally applicable direct arylations in apolar solvents. Org. Lett.10, 2299–2302 (2008). This study introduces carboxylate assistance for ruthenium-catalysed C–H activation, which allows for versatile transformations operative in apolar solvents. Google Scholar
- Lapointe, D. & Fagnou, K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett.39, 1118–1126 (2010). Google Scholar
- Biswas, B., Sugimoto, M. & Sakaki, S. C–H bond activation of benzene and methane by M(η 2 -O2CH)2 (M = Pd or Pt). A theoretical study. Organometallics19, 3895–3908 (2000). Google Scholar
- Davies, D. L., Macgregor, S. A. & McMullin, C. L. Computational studies of carboxylate-assisted C–H activation and functionalization at Group 8–10 transition metal centers. Chem. Rev.117, 8649–8709 (2017). Google Scholar
- Rogge, T., Oliveira, J. C. A., Kuniyil, R., Hu, L. & Ackermann, L. Reactivity-controlling factors in carboxylate-assisted C–H activation under 4d and 3d transition metal catalysis. ACS Catal.10, 10551–10558 (2020). Google Scholar
- Murahashi, S. Synthesis of phthalimidines from Schiff bases and carbon monoxide. J. Am. Chem. Soc.77, 6403–6404 (1955). This pioneering investigation arguably represents the first example of catalytic C–H activation, thereby laying the foundation for numerous further advances and achievements in this research area. Google Scholar
- Fujiwara, Y., Moritani, I., Danno, S., Asano, R. & Teranishi, S. Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate. J. Am. Chem. Soc.91, 7166–7169 (1969). This publication accomplishes the olefination of arenes with unactivated olefines via palladium-catalysed C–H activation for the first time. Google Scholar
- Lewis, L. N. & Smith, J. F. Catalytic carbon–carbon bond formation via ortho-metalated complexes. J. Am. Chem. Soc.108, 2728–2735 (1986). This study employsortho-metallated ruthenium complexes for the catalytic formation of C–C bonds, thus inspiring further developments in C–H activation by the action of ruthenium catalysts. Google Scholar
- Nakamura, N., Tajima, Y. & Sakai, K. Direct phenylation of isoxazoles using palladium catalysts. Synthesis of 4-phenylmuscimol. Heterocycles17, 235–245 (1982). Google Scholar
- Murai, S. et al. Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins. Nature366, 529–531 (1993). This study constitutes one of the most notable contributions to catalysed C–H activation featuring a broad applicability. ADSGoogle Scholar
- Matsubara, T., Koga, N., Musaev, D. G. & Morokuma, K. Density functional study on activation of ortho-CH bond in aromatic ketone by Ru complex. Role of unusual five-coordinated d 6 metallacycle intermediate with agostic interaction. J. Am. Chem. Soc.120, 12692–12693 (1998). Google Scholar
- Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res.48, 1053–1064 (2015). Google Scholar
- Hummel, J. R., Boerth, J. A. & Ellman, J. A. Transition-metal-catalyzed C–H bond addition to carbonyls, imines, and related polarized π bonds. Chem. Rev.117, 9163–9227 (2017). Google Scholar
- Dong, Z., Ren, Z., Thompson, S. J., Xu, Y. & Dong, G. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev.117, 9333–9403 (2017). Google Scholar
- Rej, S., Ano, Y. & Chatani, N. Bidentate directing groups: an efficient tool in C–H bond functionalization chemistry for the expedient construction of C–C bonds. Chem. Rev.120, 1788–1887 (2020). Google Scholar
- Satoh, T. & Miura, M. Oxidative coupling of aromatic substrates with alkynes and alkenes under rhodium catalysis. Chem. Eur. J.16, 11212–11222 (2010). Google Scholar
- Baudoin, O. Ring construction by palladium(0)-catalyzed C(sp 3 )–H activation. Acc. Chem. Res.50, 1114–1123 (2017). Google Scholar
- He, C., Whitehurst, W. G. & Gaunt, M. J. Palladium-catalyzed C(sp 3 )–H bond functionalization of aliphatic amines. Chem5, 1031–1058 (2019). Google Scholar
- Chen, Z. et al. Catalytic alkylation of unactivated C(sp 3 )–H bonds for C(sp 3 )–C(sp 3 ) bond formation. Chem. Soc. Rev.48, 4921–4942 (2019). Google Scholar
- Das, S., Incarvito, C. D., Crabtree, R. H. & Brudvig, G. W. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science312, 1941–1943 (2006). This publication constitutes a milestone in C–H activation chemistry, owing to the elegant design of a hydrogen-bonding linker, which non-covalently binds to the substrates and enables remote C–H activation. ADSGoogle Scholar
- Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature486, 518–522 (2012). ADSGoogle Scholar
- Yang, Y.-F. et al. Palladium-catalyzed meta-selective C–H bond activation with a nitrile-containing template: computational study on mechanism and origins of selectivity. J. Am. Chem. Soc.136, 344–355 (2014). Google Scholar
- Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta- and para-C(sp 2 )–H bonds with covalently attached directing groups. Angew. Chem. Int. Ed.58, 10820–10843 (2018). Google Scholar
- Bag, S. et al. Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc.137, 11888–11891 (2015). This publication arguably presents a directedpara-selective C–H activation, employing a carefully designed D-shaped template. Google Scholar
- Zhang, Z., Tanaka, K. & Yu, J.-Q. Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature543, 538–542 (2017). ADSGoogle Scholar
- Mihai, M. T., Genov, G. R. & Phipps, R. J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: a focus on metals other than palladium. Chem. Soc. Rev.47, 149–171 (2018). Google Scholar
- Leitch, J. A. & Frost, C. G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev.46, 7145–7153 (2017). Google Scholar
- Korvorapun, K., Kuniyil, R. & Ackermann, L. Late-stage diversification by selectivity switch in meta-C–H activation: evidence for singlet stabilization. ACS Catal.10, 435–440 (2020). Google Scholar
- Hofmann, N. & Ackermann, L. meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc.135, 5877–5884 (2013). Google Scholar
- Li, J. et al. N-Acyl amino acid ligands for ruthenium(II)-catalyzed meta-C–H tert-alkylation with removable auxiliaries. J. Am. Chem. Soc.137, 13894–13901 (2015). Google Scholar
- Catellani, M., Motti, E. & Della Ca’, N. Catalytic sequential reactions involving palladacycle-directed aryl coupling steps. Acc. Chem. Res.41, 1512–1522 (2008). Google Scholar
- Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Ed. Engl.36, 119–122 (1997). Google Scholar
- Okumura, S. et al. para-Selective alkylation of benzamides and aromatic ketones by cooperative nickel/aluminum catalysis. J. Am. Chem. Soc.138, 14699–14704 (2016). Google Scholar
- St John-Campbell, S. & Bull, J. A. Transient imines as ‘next generation’ directing groups for the catalytic functionalisation of C–H bonds in a single operation. Org. Biomol. Chem.16, 4582–4595 (2018). Google Scholar
- Gandeepan, P. & Ackermann, L. Transient directing groups for transformative C–H activation by synergistic metal catalysis. Chem4, 199–222 (2018). Google Scholar
- Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp 3 )–H bonds using a transient directing group. Science351, 252–256 (2016). ADSGoogle Scholar
- Yao, Q.-J., Zhang, S., Zhan, B.-B. & Shi, B.-F. Atroposelective synthesis of axially chiral biaryls by palladium-catalyzed asymmetric C–H olefination enabled by a transient chiral auxiliary. Angew. Chem. Int. Ed.56, 6617–6621 (2017). Google Scholar
- Liao, G. et al. Scalable, stereocontrolled formal syntheses of (+)-isoschizandrin and (+)-steganone: development and applications of palladium(II)-catalyzed atroposelective C–H alkynylation. Angew. Chem. Int. Ed.57, 3661–3665 (2018). Google Scholar
- Brown, D. G. & Bostrom, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem.59, 4443–4458 (2016). Google Scholar
- Goldberg, F. W., Kettle, J. G., Kogej, T., Perry, M. W. & Tomkinson, N. P. Designing novel building blocks is an overlooked strategy to improve compound quality. Drug Discov. Today20, 11–17 (2015). Google Scholar
- Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science363, eaat0805 (2019). Google Scholar
- Tsang, W. C., Zheng, N. & Buchwald, S. L. Combined C–H functionalization/C–N bond formation route to carbazoles. J. Am. Chem. Soc.127, 14560–14561 (2005). This article represents the first example of a palladium-catalysed direct C–H amination via C–N bond-forming reductive elimination from a carbometallation intermediate. Google Scholar
- Thu, H. Y., Yu, W. Y. & Che, C. M. Intermolecular amidation of unactivated sp 2 and sp 3 C–H bonds via palladium-catalyzed cascade C–H activation/nitrene insertion. J. Am. Chem. Soc.128, 9048–9049 (2006). This study reports the first example of an intermolecular C–H amination by oxidative palladium catalysis to showcase the utility of a carbometallation intermediate as an electrophilic amine source. Google Scholar
- Wang, Z., Ni, J., Kuninobu, Y. & Kanai, M. Copper-catalyzed intramolecular C(sp 3 )–H and C(sp 2 )–H amidation by oxidative cyclization. Angew. Chem. Int. Ed.53, 3496–3499 (2014). Google Scholar
- Chen, X., Hao, X. S., Goodhue, C. E. & Yu, J. Q. Cu(II)-catalyzed functionalizations of aryl C–H bonds using O2 as an oxidant. J. Am. Chem. Soc.128, 6790–6791 (2006). Google Scholar
- Zhang, L. B. et al. Cobalt(II)-catalyzed C–H amination of arenes with simple alkylamines. Org. Lett.18, 1318–1321 (2016). Google Scholar
- Zhao, H., Shang, Y. & Su, W. Rhodium(III)-catalyzed intermolecular N-chelator-directed aromatic C–H amidation with amides. Org. Lett.15, 5106–5109 (2013). Google Scholar
- Suzuki, C., Hirano, K., Satoh, T. & Miura, M. Direct synthesis of N–H carbazoles via iridium(III)-catalyzed intramolecular C–H amination. Org. Lett.17, 1597–1600 (2015). Google Scholar
- Yan, Q. et al. Nickel-catalyzed direct amination of arenes with alkylamines. Org. Lett.17, 2482–2485 (2015). Google Scholar
- Wu, X., Zhao, Y. & Ge, H. Nickel-catalyzed site-selective amidation of unactivated C(sp 3 )–H bonds. Chem. Eur. J.20, 9530–9533 (2014). Google Scholar
- Yang, M. et al. Silver-catalysed direct amination of unactivated C–H bonds of functionalized molecules. Nat. Commun.5, 4707 (2014). ADSGoogle Scholar
- Hartwig, J. F. Electronic effects on reductive elimination to form carbon–carbon and carbon–heteroatom bonds from palladium(II) complexes. Inorg. Chem.46, 1936–1947 (2007). Google Scholar
- Wang, F. & Stahl, S. S. Merging photochemistry with electrochemistry: functional-group tolerant electrochemical amination of C(sp 3 )–H bonds. Angew. Chem. Int. Ed.58, 6385–6390 (2019). Google Scholar
- Yang, Q. L. et al. Copper-catalyzed electrochemical C–H amination of arenes with secondary amines. J. Am. Chem. Soc.140, 11487–11494 (2018). Google Scholar
- Sauermann, N., Mei, R. & Ackermann, L. Electrochemical C–H amination by cobalt catalysis in a renewable solvent. Angew. Chem. Int. Ed.57, 5090–5094 (2018). Google Scholar
- Qiu, Y., Stangier, M., Meyer, T. H., Oliveira, J. C. A. & Ackermann, L. Iridium-catalyzed electrooxidative C–H activation by chemoselective redox-catalyst cooperation. Angew. Chem. Int. Ed.57, 14179–14183 (2018). Google Scholar
- Gao, X., Wang, P., Zeng, L., Tang, S. & Lei, A. Cobalt(II)-catalyzed electrooxidative C–H amination of arenes with alkylamines. J. Am. Chem. Soc.140, 4195–4199 (2018). Google Scholar
- Choi, S., Chatterjee, T., Choi, W. J., You, Y. & Cho, E. J. Synthesis of carbazoles by a merged visible light photoredox and palladium-catalyzed process. ACS Catal.5, 4796–4802 (2015). Google Scholar
- Tan, Y. & Hartwig, J. F. Palladium-catalyzed amination of aromatic C–H bonds with oxime esters. J. Am. Chem. Soc.132, 3676–3677 (2010). Google Scholar
- Kawano, T., Hirano, K., Satoh, T. & Miura, M. A new entry of amination reagents for heteroaromatic C–H bonds: copper-catalyzed direct amination of azoles with chloroamines at room temperature. J. Am. Chem. Soc.132, 6900–6901 (2010). Google Scholar
- Grohmann, C., Wang, H. & Glorius, F. Rh[III]-catalyzed direct C–H amination using N-chloroamines at room temperature. Org. Lett.14, 656–659 (2012). Google Scholar
- Ng, K. H., Zhou, Z. & Yu, W. Y. Rhodium(III)-catalyzed intermolecular direct amination of aromatic C–H bonds with N-chloroamines. Org. Lett.14, 272–275 (2012). Google Scholar
- Kim, J. Y. et al. Rhodium-catalyzed intermolecular amidation of arenes with sulfonyl azides via chelation-assisted C–H bond activation. J. Am. Chem. Soc.134, 9110–9113 (2012). Google Scholar
- Peng, J., Xie, Z., Chen, M., Wang, J. & Zhu, Q. Copper-catalyzed C(sp 2 )–H amidation with azides as amino sources. Org. Lett.16, 4702–4705 (2014). Google Scholar
- Sun, B., Yoshino, T., Matsunaga, S. & Kanai, M. Air-stable carbonyl(pentamethylcyclopentadienyl)cobalt diiodide complex as a precursor for cationic (pentamethylcyclopentadienyl)cobalt(III) catalysis: application for directed C-2 selective C–H amidation of indoles. Adv. Synth. Catal.356, 1491–1495 (2014). Google Scholar
- Bhanuchandra, M., Yadav, M. R., Rit, R. K., Rao Kuram, M. & Sahoo, A. K. Ru(II)-catalyzed intermolecular ortho-C–H amidation of aromatic ketones with sulfonyl azides. Chem. Commun.49, 5225–5227 (2013). Google Scholar
- Liu, B., Li, B. & Wang, B. Ru(II)-catalyzed amidation reactions of 8-methylquinolines with azides via C(sp 3 )–H activation. Chem. Commun.51, 16334–16337 (2015). Google Scholar
- Wang, H., Tang, G. & Li, X. Rhodium(III)-catalyzed amidation of unactivated C(sp 3 )–H bonds. Angew. Chem. Int. Ed.54, 13049–13052 (2015). Google Scholar
- Kang, T., Kim, Y., Lee, D., Wang, Z. & Chang, S. Iridium-catalyzed intermolecular amidation of sp 3 C–H bonds: late-stage functionalization of an unactivated methyl group. J. Am. Chem. Soc.136, 4141–4144 (2014). Google Scholar
- Ryu, J., Kwak, J., Shin, K., Lee, D. & Chang, S. Ir(III)-catalyzed mild C–H amidation of arenes and alkenes: an efficient usage of acyl azides as the nitrogen source. J. Am. Chem. Soc.135, 12861–12868 (2013). Google Scholar
- Sun, K., Li, Y., Xiong, T., Zhang, J. & Zhang, Q. Palladium-catalyzed C–H aminations of anilides with N-fluorobenzenesulfonimide. J. Am. Chem. Soc.133, 1694–1697 (2011). Google Scholar
- Zhou, B., Du, J., Yang, Y., Feng, H. & Li, Y. Rhodium-catalyzed direct addition of aryl C–H bonds to nitrosobenzenes at room temperature. Org. Lett.15, 6302–6305 (2013). Google Scholar
- Breslow, R. & Gellman, S. H. Tosylamidation of cyclohexane by a cytochrome-P-450 Model. J. Chem. Soc. Chem. Commun.https://doi.org/10.1039/C39820001400 (1982). ArticleGoogle Scholar
- Breslow, R. & Gellman, S. H. Intramolecular nitrene carbon–hydrogen insertions mediated by transition-metal complexes as nitrogen analogs of cytochrome P-450 reactions. J. Am. Chem. Soc.105, 6728–6729 (1983). Google Scholar
- Varela-Álvarez, A. et al. Rh2(II,III) catalysts with chelating carboxylate and carboxamidate supports: electronic structure and nitrene transfer reactivity. J. Am. Chem. Soc.138, 2327–2341 (2016). Google Scholar
- Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res.45, 911–922 (2012). Google Scholar
- Caballero, A. et al. Highly regioselective functionalization of aliphatic carbon–hydrogen bonds with a perbromohomoscorpionate copper(I) catalyst. J. Am. Chem. Soc.125, 1446–1447 (2003). Google Scholar
- Albone, D. P., Aujla, P. S., Challenger, S. & Derrick, A. M. A simple copper catalyst for both aziridination of alkenes and amination of activated hydrocarbons with chloramine-T trihydrate. J. Org. Chem.63, 9569–9571 (1998). Google Scholar
- Harvey, M. E., Musaev, D. G. & Du Bois, J. A diruthenium catalyst for selective, intramolecular allylic C–H amination: reaction development and mechanistic insight gained through experiment and theory. J. Am. Chem. Soc.133, 17207–17216 (2011). Google Scholar
- Ragaini, F. et al. Amination of benzylic C–H bonds by arylazides catalyzed by Co II -porphyrin complexes: a synthetic and mechanistic study. Chem. Eur. J.9, 249–259 (2003). Google Scholar
- Paradine, S. M. et al. A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp 3 )–H amination. Nat. Chem.7, 987–994 (2015). Google Scholar
- Yu, X. Q., Huang, J. S., Zhou, X. G. & Che, C. M. Amidation of saturated C–H bonds catalyzed by electron-deficient ruthenium and manganese porphyrins. A highly catalytic nitrogen atom transfer process. Org. Lett.2, 2233–2236 (2000). Google Scholar
- Aguila, M. J., Badiei, Y. M. & Warren, T. H. Mechanistic insights into C–H amination via dicopper nitrenes. J. Am. Chem. Soc.135, 9399–9406 (2013). Google Scholar
- Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science359, 1016–1021 (2018). ADSGoogle Scholar
- Hwang, Y., Jung, H., Lee, E., Kim, D. & Chang, S. Quantitative analysis on two-point ligand modulation of iridium catalysts for chemodivergent C–H amidation. J. Am. Chem. Soc.142, 8880–8889 (2020). Google Scholar
- Park, Y. & Chang, S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat. Catal.2, 219–227 (2019). This investigation achieves the challenging construction of valuable γ-lactams in an asymmetric fashion under iridium catalysis, employing a novel chiral hydrogen-bonding ligand. Google Scholar
- Lee, J. et al. Versatile Cp*Co(III)(LX) catalyst system for selective intramolecular C–H amidation reactions. J. Am. Chem. Soc.142, 12324–12332 (2020). Google Scholar
- Jung, H. et al. Harnessing secondary coordination sphere interactions that enable the selective amidation of benzylic C–H bonds. J. Am. Chem. Soc.141, 15356–15366 (2019). Google Scholar
- Liang, Y.-F. & Jiao, N. Oxygenation via C–H/C–C bond activation with molecular oxygen. Acc. Chem. Res.50, 1640–1653 (2017). Google Scholar
- Thirunavukkarasu, V. S., Kozhushkov, S. I. & Ackermann, L. C–H nitrogenation and oxygenation by ruthenium catalysis. Chem. Commun.50, 29–39 (2014). Google Scholar
- Caballero, A. & Pérez, P. J. Methane as raw material in synthetic chemistry: the final frontier. Chem. Soc. Rev.42, 8809–8820 (2013). Google Scholar
- Gol’dshleger, N. F., Khidekel, M. L., Shilov, A. E. & Shteinman, A. A. Oxidative dehydrogenation of saturated hydrocarbons in palladium(II) complex solutions. Kinet. Katal.15, 261 (1974). Google Scholar
- Jintoku, T., Nishimura, K., Takaki, K. & Fujiwara, Y. Palladium catalyzed transformation of benzene to phenol with molecular oxygen. Chem. Lett.19, 1687–1688 (1990). Google Scholar
- Dick, A. R., Hull, K. L. & Sanford, M. S. A highly selective catalytic method for the oxidative functionalization of C–H bonds. J. Am. Chem. Soc.126, 2300–2301 (2004). This contribution demonstrates the selective formation of C–O bonds via palladium-catalysed C(sp2)–H and challenging C(sp3)–H activation. Google Scholar
- Giri, R. et al. Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions: a dual role for carboxylic anhydrides in catalytic C–H bond oxidation. Angew. Chem. Int. Ed.44, 7420–7424 (2005). Google Scholar
- Thirunavukkarasu, V. S., Hubrich, J. & Ackermann, L. Ruthenium-catalyzed oxidative C(sp 2 )–H bond hydroxylation: site-selective C–O bond formation on benzamides. Org. Lett.14, 4210–4213 (2012). Google Scholar
- Shan, G., Han, X., Lin, Y., Yu, S. & Rao, Y. Broadening the catalyst and reaction scope of regio- and chemoselective C–H oxygenation: a convenient and scalable approach to 2-acylphenols by intriguing Rh(II) and Ru(II) catalysis. Org. Biomol. Chem.11, 2318–2322 (2013). Google Scholar
- Wang, Z., Kuninobu, Y. & Kanai, M. Copper-mediated direct C(sp 3 )–H and C(sp 2 )–H acetoxylation. Org. Lett.16, 4790–4793 (2014). Google Scholar
- Bhadra, S., Dzik, W. I. & Gooßen, L. J. Synthesis of aryl ethers from benzoates through carboxylate-directed C–H-activating alkoxylation with concomitant protodecarboxylation. Angew. Chem. Int. Ed.52, 2959–2962 (2013). Google Scholar
- Ueno, R., Natsui, S. & Chatani, N. Cobalt(II)-catalyzed acyloxylation of C–H bonds in aromatic amides with carboxylic acids. Org. Lett.20, 1062–1065 (2018). Google Scholar
- Yang, F., Zhang, H., Liu, X., Wang, B. & Ackermann, L. Transition metal-catalyzed regio-selective aromatic C–H bond oxidation for C–O bond formation. Chin. J. Org. Chem.39, 59 (2019). Google Scholar
- Kim, J., Shin, K., Jin, S., Kim, D. & Chang, S. Oxidatively induced reductive elimination: exploring the scope and catalyst systems with Ir, Rh, and Ru complexes. J. Am. Chem. Soc.141, 4137–4146 (2019). Google Scholar
- Li, L., Brennessel, W. W. & Jones, W. D. An efficient low-temperature route to polycyclic isoquinoline salt synthesis via C–H activation with [Cp*MCl2]2 (M = Rh, Ir). J. Am. Chem. Soc.130, 12414–12419 (2008). Google Scholar
- Wendlandt, A. E., Suess, A. M. & Stahl, S. S. Copper-catalyzed aerobic oxidative C–H functionalizations: trends and mechanistic insights. Angew. Chem. Int. Ed.50, 11062–11087 (2011). Google Scholar
- Zhang, Y.-H. & Yu, J.-Q. Pd(II)-catalyzed hydroxylation of arenes with 1 atm of O2 or air. J. Am. Chem. Soc.131, 14654–14655 (2009). Google Scholar
- Yan, Y. et al. PdCl2 and N-hydroxyphthalimide co-catalyzed C–H hydroxylation by dioxygen activation. Angew. Chem. Int. Ed.52, 5827–5831 (2013). ADSGoogle Scholar
- Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev.110, 1147–1169 (2010). Google Scholar
- Canty, A. J., Denney, M. C., van Koten, G., Skelton, B. W. & White, A. H. Carbon–oxygen bond formation at metal(IV) centers: reactivity of palladium(II) and platinum(II) complexes of the [2,6-(dimethylaminomethyl)phenyl-N,C,N]-(pincer) ligand toward iodomethane and dibenzoyl peroxide; structural studies of M(II) and M(IV) complexes. Organometallics23, 5432–5439 (2004). Google Scholar
- Massignan, L. et al. C–H oxygenation reactions enabled by dual catalysis with electrogenerated hypervalent iodine species and ruthenium complexes. Angew. Chem. Int. Ed.59, 3184–3189 (2020). Google Scholar
- Sauer, G. S. & Lin, S. An electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal.8, 5175–5187 (2018). Google Scholar
- Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed.57, 5594–5619 (2018). Google Scholar
- Kalsi, D., Dutta, S., Barsu, N., Rueping, M. & Sundararaju, B. Room-temperature C–H bond functionalization by merging cobalt and photoredox catalysis. ACS Catal.8, 8115–8120 (2018). Google Scholar
- Zhang, S.-K., Struwe, J., Hu, L. & Ackermann, L. Nickela-electrocatalyzed C–H alkoxylation with secondary alcohols: oxidation-induced reductive elimination at nickel(III). Angew. Chem. Int. Ed.59, 3178–3183 (2020). Google Scholar
- Sauermann, N., Meyer, T. H., Tian, C. & Ackermann, L. Electrochemical cobalt-catalyzed C–H oxygenation at room temperature. J. Am. Chem. Soc.139, 18452–18455 (2017). This contribution achieves C–H oxygenation under cobalt catalysis, employing electricity as the environmentally benign oxidant. Google Scholar
- Meyer, T. H., Oliveira, J. C. A., Ghorai, D. & Ackermann, L. Insights into cobalta(III/IV/II)-electrocatalysis: oxidation-induced reductive elimination for twofold C–H activation. Angew. Chem. Int. Ed.59, 10955–10960 (2020). Google Scholar
- Waltz, K. M., He, X., Muhoro, C. & Hartwig, J. F. Hydrocarbon functionalization by transition metal boryls. J. Am. Chem. Soc.117, 11357–11358 (1995). Google Scholar
- Waltz, K. M. & Hartwig, J. F. Selective functionalization of alkanes by transition-metal boryl complexes. Science277, 211–213 (1997). Google Scholar
- Iverson, C. N. & Smith, M. R. Stoichiometric and catalytic B–C bond formation from unactivated hydrocarbons and boranes. J. Am. Chem. Soc.121, 7696–7697 (1999). Google Scholar
- Ishiyama, T. et al. Mild iridium-catalyzed borylation of arenes. high turnover numbers, room temperature reactions, and isolation of a potential intermediate. J. Am. Chem. Soc.124, 390–391 (2002). Google Scholar
- Cho, J.-Y., Tse, M. K., Holmes, D., Maleczka, R. E. & Smith, M. R. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science295, 305–308 (2002). ADSGoogle Scholar
- Preshlock, S. M. et al. High-throughput optimization of Ir-catalyzed C–H borylation: a tutorial for practical applications. J. Am. Chem. Soc.135, 7572–7582 (2013). Google Scholar
- Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev.40, 1992–2002 (2011). Google Scholar
- Boller, T. M. et al. Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. Intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc.127, 14263–14278 (2005). Google Scholar
- Press, L. P., Kosanovich, A. J., McCulloch, B. J. & Ozerov, O. V. High-turnover aromatic C–H borylation catalyzed by POCOP-type pincer complexes of iridium. J. Am. Chem. Soc.138, 9487–9497 (2016). Google Scholar
- Zhu, L. et al. Ir(III)/Ir(V) or Ir(I)/Ir(III) catalytic cycle? Steric-effect-controlled mechanism for the para-C–H borylation of arenes. Organometallics36, 2107–2115 (2017). Google Scholar
- Zhong, R.-L. & Sakaki, S. sp 3 C–H borylation catalyzed by iridium(III) triboryl complex: comprehensive theoretical study of reactivity, regioselectivity, and prediction of excellent ligand. J. Am. Chem. Soc.141, 9854–9866 (2019). Google Scholar
- Shi, Y., Gao, Q. & Xu, S. Chiral bidentate boryl ligand enabled iridium-catalyzed enantioselective C(sp 3 )–H borylation of cyclopropanes. J. Am. Chem. Soc.141, 10599–10604 (2019). Google Scholar
- Jones, M. R., Fast, C. D. & Schley, N. D. Iridium-catalyzed sp 3 C–H borylation in hydrocarbon solvent enabled by 2,2′-dipyridylarylmethane ligands. J. Am. Chem. Soc.142, 6488–6492 (2020). Google Scholar
- Cook, A. K., Schimler, S. D., Matzger, A. J. & Sanford, M. S. Catalyst-controlled selectivity in the C–H borylation of methane and ethane. Science351, 1421–1424 (2016). ADSGoogle Scholar
- Smith, K. T. et al. Catalytic borylation of methane. Science351, 1424–1427 (2016). ADSGoogle Scholar
- Ahn, S. et al. Rational design of a catalyst for the selective monoborylation of methane. ACS Catal.8, 10021–10031 (2018). Google Scholar
- Obligacion, J. V., Semproni, S. P. & Chirik, P. J. Cobalt-catalyzed C–H borylation. J. Am. Chem. Soc.136, 4133–4136 (2014). Google Scholar
- Obligacion, J. V., Semproni, S. P., Pappas, I. & Chirik, P. J. Cobalt-catalyzed C(sp 2 )-H borylation: mechanistic insights inspire catalyst design. J. Am. Chem. Soc.138, 10645–10653 (2016). Google Scholar
- Obligacion, J. V. & Chirik, P. J. Mechanistic studies of cobalt-catalyzed C(sp 2 )–H borylation of five-membered heteroarenes with pinacolborane. ACS Catal.7, 4366–4371 (2017). Google Scholar
- Genov, G. R., Douthwaite, J. L., Lahdenperä, A. S. K., Gibson, D. C. & Phipps, R. J. Enantioselective remote C–H activation directed by a chiral cation. Science367, 1246–1251 (2020). ADSGoogle Scholar
- Kuninobu, Y., Ida, H., Nishi, M. & Kanai, M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem.7, 712–717 (2015). Google Scholar
- Petrone, D. A., Ye, J. & Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev.116, 8003–8104 (2016). Google Scholar
- Giri, R., Chen, X. & Yu, J.-Q. Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew. Chem. Int. Ed.44, 2112–2115 (2005). Google Scholar
- Hull, K. L., Anani, W. Q. & Sanford, M. S. Palladium-catalyzed fluorination of carbon-hydrogen bonds. J. Am. Chem. Soc.128, 7134–7135 (2006). Google Scholar
- Alsters, P. L. et al. Rigid five- and six-membered C,N,N′-bound aryl-, benzyl-, and alkylorganopalladium complexes: sp 2 vs. sp 3 carbon-hydrogen activation during cyclopalladation and palladium(IV) intermediates in oxidative addition reactions with dihalogens and alkyl halides. Organometallics12, 1831–1844 (1993). Google Scholar
- Newkome, G. R., Puckett, W. E., Gupta, V. K. & Kiefer, G. E. Cyclometalation of the platinum metals with nitrogen and alkyl, alkenyl, and benzyl carbon donors. Chem. Rev.86, 451–489 (1986). Google Scholar
- McMurtrey, K. B., Racowski, J. M. & Sanford, M. S. Pd-catalyzed C–H fluorination with nucleophilic fluoride. Org. Lett.14, 4094–4097 (2012). Google Scholar
- Furuya, T., Kamlet, A. S. & Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature473, 470–477 (2011). ADSGoogle Scholar
- Grimme, S., Hansen, A., Brandenburg, J. G. & Bannwarth, C. Dispersion-corrected mean-field electronic structure methods. Chem. Rev.116, 5105–5154 (2016). Google Scholar
- Sperger, T., Sanhueza, I. A., Kalvet, I. & Schoenebeck, F. Computational studies of synthetically relevant homogeneous organometallic catalysis involving Ni, Pd, Ir, and Rh: an overview of commonly employed DFT methods and mechanistic insights. Chem. Rev.115, 9532–9586 (2015). Google Scholar
- Musaev, D. G., Figg, T. M. & Kaledin, A. L. Versatile reactivity of Pd-catalysts: mechanistic features of the mono-N-protected amino acid ligand and cesium-halide base in Pd-catalyzed C–H bond functionalization. Chem. Soc. Rev.43, 5009–5031 (2014). Google Scholar
- Bartlett, R. J. & Musiał, M. Coupled-cluster theory in quantum chemistry. Rev. Mod. Phys.79, 291–352 (2007). ADSGoogle Scholar
- Riplinger, C., Pinski, P., Becker, U., Valeev, E. F. & Neese, F. Sparse maps—a systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys.144, 024109 (2016). ADSGoogle Scholar
- Bursch, M. et al. Understanding and quantifying London dispersion effects in organometallic complexes. Acc. Chem. Res.52, 258–266 (2019). Google Scholar
- Zhao, Y. & Truhlar, D. G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys.125, 194101 (2006). ADSGoogle Scholar
- Casanova-Páez, M., Dardis, M. B. & Goerigk, L. ωB2PLYP and ωB2GPPLYP: the first two double-hybrid density functionals with long-range correction optimized for excitation energies. J. Chem. Theory Comput.15, 4735–4744 (2019). Google Scholar
- Mennucci, B. & Tomasi, J. Continuum solvation models: a new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys.106, 5151–5158 (1997). ADSGoogle Scholar
- Pyykkö, P. Relativistic effects in chemistry: more common than you thought. Annu. Rev. Phys. Chem.63, 45–64 (2012). ADSGoogle Scholar
- Küchle, W., Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys.100, 7535–7542 (1994). ADSGoogle Scholar
- Hay, P. J. & Martin, R. L. Theoretical studies of the structures and vibrational frequencies of actinide compounds using relativistic effective core potentials with Hartree–Fock and density functional methods: UF6, NpF6, and PuF6. J. Chem. Phys.109, 3875–3881 (1998). ADSGoogle Scholar
- Pantazis, D. A., Chen, X.-Y., Landis, C. R. & Neese, F. All-electron scalar relativistic basis sets for third-row transition metal atoms. J. Chem. Theory Comput.4, 908–919 (2008). Google Scholar
- Senn, H. M. & Thiel, W. QM/MM methods for biomolecular systems. Angew. Chem. Int. Ed.48, 1198–1229 (2009). Google Scholar
- Dapprich, S., Komáromi, I., Byun, K. S., Morokuma, K. & Frisch, M. J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM461–462, 1–21 (1999). Google Scholar
- Maseras, F. & Morokuma, K. IMOMM: a new integrated ab initio+molecular mechanics geometry optimization scheme of equilibrium structures and transition states. J. Comput. Chem.16, 1170–1179 (1995). Google Scholar
- Burke, K., Werschnik, J. & Gross, E. K. U. Time-dependent density functional theory: past, present, and future. J. Chem. Phys.123, 062206 (2005). ADSGoogle Scholar
- Finley, J., Malmqvist, P.-Å., Roos, B. O. & Serrano-Andrés, L. The multi-state CASPT2 method. Chem. Phys. Lett.288, 299–306 (1998). ADSGoogle Scholar
- Houk, K. N. & Liu, F. Holy grails for computational organic chemistry and biochemistry. Acc. Chem. Res.50, 539–543 (2017). Google Scholar
- Besora, M. et al. A quantitative model for alkane nucleophilicity based on C–H bond structural/topological descriptors. Angew. Chem. Int. Ed.59, 3112–3116 (2020). Google Scholar
- McLarney, B. D., Hanna, S., Musaev, D. G. & France, S. Predictive model for the [Rh2(esp)2]-catalyzed intermolecular C(sp 3 )–H bond insertion of β-carbonyl ester carbenes: interplay between theory and experiment. ACS Catal.9, 4526–4538 (2019). Google Scholar
- Zimmerman, P. M. Automated discovery of chemically reasonable elementary reaction steps. J. Comput. Chem.34, 1385–1392 (2013). Google Scholar
- Maeda, S., Harabuchi, Y., Takagi, M., Taketsugu, T. & Morokuma, K. Artificial force induced reaction (AFIR) method for exploring quantum chemical potential energy surfaces. Chem. Rec.16, 2232–2248 (2016). Google Scholar
- Reyes, R. L. et al. Asymmetric remote C–H borylation of aliphatic amides and esters with a modular iridium catalyst. Science369, 970–974 (2020). ADSGoogle Scholar
- Zahrt, A. F. et al. Prediction of higher-selectivity catalysts by computer-driven workflow and machine learning. Science363, eaau5631 (2019). Google Scholar
- Newton, C. G., Wang, S.-G., Oliveira, C. C. & Cramer, N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev.117, 8908–8976 (2017). Google Scholar
- Giri, R., Shi, B.-F., Engle, K. M., Maugel, N. & Yu, J.-Q. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem. Soc. Rev.38, 3242–3272 (2009). Google Scholar
- O’Malley, S. J., Tan, K. L., Watzke, A., Bergman, R. G. & Ellman, J. A. Total synthesis of (+)-lithospermic acid by asymmetric intramolecular alkylation via catalytic C–H bond activation. J. Am. Chem. Soc.127, 13496–13497 (2005). Google Scholar
- Kakiuchi, F., Le Gendre, P., Yamada, A., Ohtaki, H. & Murai, S. Atropselective alkylation of biaryl compounds by means of transition metal-catalyzed C–H/olefin coupling. Tetrahedron Asymmetry11, 2647–2651 (2000). Google Scholar
- Wencel-Delord, J. & Colobert, F. Asymmetric C(sp 2 )–H activation. Chem. Eur. J.19, 14010–14017 (2013). Google Scholar
- Shi, B.-F., Maugel, N., Zhang, Y.-H. & Yu, J.-Q. PdII-catalyzed enantioselective activation of C(sp 2 )–H and C(sp 3 )–H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed.47, 4882–4886 (2008). Google Scholar
- Shi, B.-F., Zhang, Y.-H., Lam, J. K., Wang, D.-H. & Yu, J.-Q. Pd(II)-catalyzed enantioselective C–H olefination of diphenylacetic acids. J. Am. Chem. Soc.132, 460–461 (2010). Google Scholar
- Laforteza, B. N., Chan, K. S. L. & Yu, J.-Q. Enantioselective ortho-C–H cross-coupling of diarylmethylamines with organoborons. Angew. Chem. Int. Ed.54, 11143–11146 (2015). Google Scholar
- Diesel, J. & Cramer, N. Generation of heteroatom stereocenters by enantioselective C–H functionalization. ACS Catal.9, 9164–9177 (2019). Google Scholar
- Albicker, M. R. & Cramer, N. Enantioselective palladium-catalyzed direct arylations at ambient temperature: access to indanes with quaternary stereocenters. Angew. Chem. Int. Ed.48, 9139–9142 (2009). Google Scholar
- Saget, T. & Cramer, N. Enantioselective C–H arylation strategy for functionalized dibenzazepinones with quaternary stereocenters. Angew. Chem. Int. Ed.52, 7865–7868 (2013). Google Scholar
- Shintani, R., Otomo, H., Ota, K. & Hayashi, T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enantioselective C–H bond functionalization. J. Am. Chem. Soc.134, 7305–7308 (2012). Google Scholar
- Li, Z., Lin, Z.-Q., Yan, C.-G. & Duan, W.-L. Pd-catalyzed asymmetric C–H bond activation for the synthesis of P-stereogenic dibenzophospholes. Organometallics38, 3916–3920 (2019). Google Scholar
- Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun.55, 8514–8523 (2019). Google Scholar
- Hazra, C. K., Dherbassy, Q., Wencel-Delord, J. & Colobert, F. Synthesis of axially chiral biaryls through sulfoxide-directed asymmetric mild C–H activation and dynamic kinetic resolution. Angew. Chem. Int. Ed.53, 13871–13875 (2014). Google Scholar
- Zheng, J. & You, S.-L. Construction of axial chirality by rhodium-catalyzed asymmetric dehydrogenative Heck coupling of biaryl compounds with alkenes. Angew. Chem. Int. Ed.53, 13244–13247 (2014). Google Scholar
- Liao, G., Zhang, T., Lin, Z.-K. & Shi, B.-F. Transition metal-catalyzed enantioselective C–H functionalization via chiral transient directing group strategies. Angew. Chem. Int. Ed.59, 19773–19786 (2020). Google Scholar
- Dhawa, U. et al. Enantioselective pallada-electrocatalyzed C–H activation by transient directing groups: expedient access to helicenes. Angew. Chem. Int. Ed.59, 13451–13457 (2020). Google Scholar
- Yamaguchi, K., Yamaguchi, J., Studer, A. & Itami, K. Hindered biaryls by C–H coupling: bisoxazoline-Pd catalysis leading to enantioselective C–H coupling. Chem. Sci.3, 2165–2169 (2012). Google Scholar
- Dherbassy, Q., Djukic, J.-P., Wencel-Delord, J. & Colobert, F. Two stereoinduction events in one C–H activation step: a route towards terphenyl ligands with two atropisomeric axes. Angew. Chem. Int. Ed.57, 4668–4672 (2018). Google Scholar
- Nguyen, Q.-H., Guo, S.-M., Royal, T., Baudoin, O. & Cramer, N. Intermolecular Palladium(0)-catalyzed atropo-enantioselective C–H arylation of heteroarenes. J. Am. Chem. Soc.142, 2161–2167 (2020). Google Scholar
- Ye, B. & Cramer, N. Chiral cyclopentadienyl ligands as stereocontrolling element in asymmetric C–H functionalization. Science338, 504–506 (2012). ADSGoogle Scholar
- Hyster, T. K., Knörr, L., Ward, T. R. & Rovis, T. Biotinylated Rh(III) complexes in engineered streptavidin for accelerated asymmetric C–H activation. Science338, 500–503 (2012). ADSGoogle Scholar
- Coulter, M. M., Dornan, P. K. & Dong, V. M. Rh-catalyzed intramolecular olefin hydroacylation: enantioselective synthesis of seven- and eight-membered heterocycles. J. Am. Chem. Soc.131, 6932–6933 (2009). Google Scholar
- Woźniak, Ł. & Cramer, N. Enantioselective C–H bond functionalizations by 3d transition-metal catalysts. Trends Chem.1, 471–484 (2019). Google Scholar
- Loup, J. et al. Asymmetric iron-catalyzed C–H alkylation enabled by remote ligand meta-substitution. Angew. Chem. Int. Ed.56, 14197–14201 (2017). Google Scholar
- Yang, J. & Yoshikai, N. Cobalt-catalyzed enantioselective intramolecular hydroacylation of ketones and olefins. J. Am. Chem. Soc.136, 16748–16751 (2014). This publication achieves intramolecular hydroacylation in an asymmetric fashion employing a low-valent cobalt catalyst bearing a chiral diphosphine ligand. Google Scholar
- Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp 3 )–H bond activation by chiral transition metal catalysts. Science359, eaao4798 (2018). Google Scholar
- Nakanishi, M., Katayev, D., Besnard, C. & Kündig, E. P. Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew. Chem. Int. Ed.50, 7438–7441 (2011). Google Scholar
- Martin, N., Pierre, C., Davi, M., Jazzar, R. & Baudoin, O. Diastereo- and enantioselective intramolecular C(sp 3 )-H arylation for the synthesis of fused cyclopentanes. Chem. Eur. J.18, 4480–4484 (2012). Google Scholar
- Saget, T. & Cramer, N. Palladium(0)-catalyzed enantioselective C–H arylation of cyclopropanes: efficient access to functionalized tetrahydroquinolines. Angew. Chem. Int. Ed.51, 12842–12845 (2012). Google Scholar
- Pedroni, J. & Cramer, N. TADDOL-based phosphorus(III)-ligands in enantioselective Pd(0)-catalysed C–H functionalisations. Chem. Commun.51, 17647–17657 (2015). Google Scholar
- Shao, Q., Wu, K., Zhuang, Z., Qian, S. & Yu, J.-Q. From Pd(OAc)2 to chiral catalysts: the discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C–H functionalization reactions. Acc. Chem. Res.53, 833–851 (2020). Google Scholar
- Sokolov, V. I., Troitskaya, L. L. & Reutov, O. A. Asymmetric cyclopalladation of dimethylaminomethylferrocene. J. Organomet. Chem.182, 537–546 (1979). This pioneering contribution achieves the asymmetric cyclopalladation of ferrocene derivatives. Google Scholar
- Hu, L. et al. PdII-catalyzed enantioselective C(sp 3 )–H activation/cross-coupling reactions of free carboxylic acids. Angew. Chem. Int. Ed.58, 2134–2138 (2019). Google Scholar
- Chen, G. et al. Ligand-accelerated enantioselective methylene C(sp 3 )–H bond activation. Science353, 1023–1027 (2016). ADSGoogle Scholar
- Reyes, R. L., Sato, M., Iwai, T. & Sawamura, M. Asymmetric synthesis of α-aminoboronates via rhodium-catalyzed enantioselective C(sp 3 )–H borylation. J. Am. Chem. Soc.142, 589–597 (2020). Google Scholar
- Park, H. S., Fan, Z., Zhu, R.-Y. & Yu, J.-Q. Distal γ-C(sp 3 )–H olefination of ketone derivatives and free carboxylic acids. Angew. Chem. Int. Ed.59, 12853–12859 (2020). Google Scholar
- Fukagawa, S. et al. Enantioselective C(sp 3 )–H amidation of thioamides catalyzed by a cobalt III /chiral carboxylic acid hybrid system. Angew. Chem. Int. Ed.58, 1153–1157 (2019). This study accomplishes challenging asymmetric amidations of C(sp3)–H bonds via Earth-abundant cobalt/chiral carboxylic acid catalysis. Google Scholar
- Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem.3, 347–360 (2019). Google Scholar
- Johnson, J. A. & Sames, D. C–H bond activation of hydrocarbon segments in complex organic molecules: total synthesis of the antimitotic rhazinilam. J. Am. Chem. Soc.122, 6321–6322 (2000). This study sets the stage for applications of C–H activations to natural product and total syntheses. Google Scholar
- Dangel, B. D., Godula, K., Youn, S. W., Sezen, B. & Sames, D. C–C bond formation via C–H bond activation: synthesis of the core of teleocidin B4. J. Am. Chem. Soc.124, 11856–11857 (2002). Google Scholar
- Li, J., Zhang, X. & Renata, H. Asymmetric chemoenzymatic synthesis of (–)-podophyllotoxin and related aryltetralin lignans. Angew. Chem. Int. Ed.58, 11657–11660 (2019). Google Scholar
- Potter, T. J. & Ellman, J. A. Total synthesis of (+)-pancratistatin by the Rh(III)-catalyzed addition of a densely functionalized benzamide to a sugar-derived nitroalkene. Org. Lett.19, 2985–2988 (2017). Google Scholar
- Xu, X., Liu, Y. & Park, C.-M. Rhodium(III)-catalyzed intramolecular annulation through C–H activation: total synthesis of (±)-antofine, (±)-septicine, (±)-tylophorine, and rosettacin. Angew. Chem. Int. Ed.51, 9372–9376 (2012). Google Scholar
- Chapman, L. M., Beck, J. C., Wu, L. & Reisman, S. E. Enantioselective total synthesis of (+)-psiguadial B. J. Am. Chem. Soc.138, 9803–9806 (2016). Google Scholar
- Ye, Q., Qu, P. & Snyder, S. A. Total syntheses of scaparvins B, C, and D enabled by a key C–H functionalization. J. Am. Chem. Soc.139, 18428–18431 (2017). Google Scholar
- Hung, K., Condakes, M. L., Morikawa, T. & Maimone, T. J. Oxidative entry into the Illicium sesquiterpenes: enantiospecific synthesis of (+)-pseudoanisatin. J. Am. Chem. Soc.138, 16616–16619 (2016). Google Scholar
- Siler, D. A., Mighion, J. D. & Sorensen, E. J. An enantiospecific synthesis of jiadifenolide. Angew. Chem. Int. Ed.53, 5332–5335 (2014). Google Scholar
- Leal, R. A. et al. Application of a palladium-catalyzed C–H functionalization/indolization method to syntheses of cis-trikentrin A and herbindole B. Angew. Chem. Int. Ed.55, 11824–11828 (2016). Google Scholar
- Fox, J. C., Gilligan, R. E., Pitts, A. K., Bennett, H. R. & Gaunt, M. J. The total synthesis of K-252c (staurosporinone) via a sequential C–H functionalisation strategy. Chem. Sci.7, 2706–2710 (2016). Google Scholar
- Lindovska, P. & Movassaghi, M. Concise synthesis of (–)-hodgkinsine, (–)-calycosidine, (–)-hodgkinsine B, (–)-quadrigemine C, and (–)-psycholeine via convergent and directed modular assembly of cyclotryptamines. J. Am. Chem. Soc.139, 17590–17596 (2017). Google Scholar
- Simmons, E. M. & Hartwig, J. F. Catalytic functionalization of unactivated primary C–H bonds directed by an alcohol. Nature483, 70–73 (2012). ADSGoogle Scholar
- Berger, M., Knittl-Frank, C., Bauer, S., Winter, G. & Maulide, N. Application of relay C–H oxidation logic to polyhydroxylated oleanane triterpenoids. Chem6, 1183–1189 (2020). Google Scholar
- Quinn, R. K. et al. Site-selective aliphatic C–H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc.138, 696–702 (2016). Google Scholar
- Hong, B. et al. Enantioselective total synthesis of (–)-incarviatone A. J. Am. Chem. Soc.137, 11946–11949 (2015). Google Scholar
- Feng, Y. et al. Total synthesis of verruculogen and fumitremorgin a enabled by ligand-controlled C–H borylation. J. Am. Chem. Soc.137, 10160–10163 (2015). Google Scholar
- Fischer, D. F. & Sarpong, R. Total synthesis of (+)-complanadine a using an iridium-catalyzed pyridine C–H functionalization. J. Am. Chem. Soc.132, 5926–5927 (2010). Google Scholar
- Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science368, 736–741 (2020). ADSGoogle Scholar
- Cherney, E. C., Lopchuk, J. M., Green, J. C. & Baran, P. S. A unified approach to ent-atisane diterpenes and related alkaloids: synthesis of (–)-methyl atisenoate, (–)-isoatisine, and the hetidine skeleton. J. Am. Chem. Soc.136, 12592–12595 (2014). Google Scholar
- Renata, H. et al. Development of a concise synthesis of ouabagenin and hydroxylated corticosteroid analogues. J. Am. Chem. Soc.137, 1330–1340 (2015). Google Scholar
- Xue, F. et al. Formal total syntheses of (–)- and (+)-actinophyllic acid. J. Org. Chem.83, 754–764 (2018). Google Scholar
- Furst, L., Narayanam, J. M. R. & Stephenson, C. R. J. Total synthesis of (+)-gliocladin C enabled by visible-light photoredox catalysis. Angew. Chem. Int. Ed.50, 9655–9659 (2011). Google Scholar
- Rosen, B. R., Werner, E. W., O’Brien, A. G. & Baran, P. S. Total synthesis of dixiamycin B by electrochemical oxidation. J. Am. Chem. Soc.136, 5571–5574 (2014). Google Scholar
- Loskot, S. A., Romney, D. K., Arnold, F. H. & Stoltz, B. M. Enantioselective total synthesis of nigelladine a via late-stage C–H oxidation enabled by an engineered P450 enzyme. J. Am. Chem. Soc.139, 10196–10199 (2017). Google Scholar
- de Rond, T. et al. Oxidative cyclization of prodigiosin by an alkylglycerol monooxygenase-like enzyme. Nat. Chem. Biol.13, 1155–1157 (2017). Google Scholar
- Zwick, C. R. & Renata, H. Evolution of biocatalytic and chemocatalytic C–H functionalization strategy in the synthesis of manzacidin C. J. Org. Chem.83, 7407–7415 (2018). Google Scholar
- Zhang, Y., Zhang, H., Ghosh, D. & Williams, R. O. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm.587, 119491–119491 (2020). Google Scholar
- Brandhofer, T. & García Mancheño, O. Site-selective C–H bond activation/functionalization of α-amino acids and peptide-like derivatives. Eur. J. Org. Chem.2018, 6050–6067 (2018). Google Scholar
- Wang, W., Lorion, M. M., Shah, J., Kapdi, A. R. & Ackermann, L. Late-stage peptide diversification by position-selective C–H activation. Angew. Chem. Int. Ed.57, 14700–14717 (2018). Google Scholar
- Sengupta, S. & Mehta, G. Macrocyclization via C–H functionalization: a new paradigm in macrocycle synthesis. Org. Biomol. Chem.18, 1851–1876 (2020). Google Scholar
- Ruiz-Rodriguez, J., Albericio, F. & Lavilla, R. Postsynthetic modification of peptides: chemoselective C-arylation of tryptophan residues. Chem. Eur. J.16, 1124–1127 (2010). Google Scholar
- Dong, H., Limberakis, C., Liras, S., Price, D. & James, K. Peptidic macrocyclization via palladium-catalyzed chemoselective indole C-2 arylation. Chem. Commun.48, 11644–11646 (2012). Google Scholar
- Zhu, Y., Bauer, M. & Ackermann, L. Late-stage peptide diversification by bioorthogonal catalytic C–H arylation at 23 °C in H2O. Chem. Eur. J.21, 9980–9983 (2015). Google Scholar
- Reay, A. J. et al. Mild and regioselective Pd(OAc)2-catalyzed C–H arylation of tryptophans by [ArN2]X, promoted by tosic acid. ACS Catal.7, 5174–5179 (2017). Google Scholar
- Mendive-Tapia, L. et al. New peptide architectures through C–H activation stapling between tryptophan–phenylalanine/tyrosine residues. Nat. Commun.6, 7160 (2015). ADSGoogle Scholar
- Perez-Rizquez, C., Abian, O. & Palomo, J. M. Site-selective modification of tryptophan and protein tryptophan residues through PdNP bionanohybrid-catalysed C–H activation in aqueous media. Chem. Commun.55, 12928–12931 (2019). This publication represents the forefront of the application of C–H activation to peptide modification, detailing the site-selective C–H activation of one or two Trp residues in the protein Cal-B in aqueous media at room temperature using a palladium nanoparticle biohybrid catalyst. Google Scholar
- Hansen, M. B., Hubálek, F., Skrydstrup, T. & Hoeg-Jensen, T. Chemo- and regioselective ethynylation of tryptophan-containing peptides and proteins. Chem. Eur. J.22, 1572–1576 (2016). Google Scholar
- Tolnai, G. L., Brand, J. P. & Waser, J. Gold-catalyzed direct alkynylation of tryptophan in peptides using TIPS-EBX. Beilstein. J. Org. Chem.12, 745–749 (2016). Google Scholar
- Bai, Z., Cai, C., Yu, Z. & Wang, H. Backbone-enabled directional peptide macrocyclization through late-stage palladium-catalyzed δ-C(sp 2 )–H olefination. Angew. Chem. Int. Ed.57, 13912–13916 (2018). Google Scholar
- Bai, Z., Cai, C., Sheng, W., Ren, Y. & Wang, H. Late-stage peptide macrocyclization by palladium-catalyzed site-selective C–H olefination of tryptophan. Angew. Chem. Int. Ed.59, 14686–14692 (2020). Google Scholar
- Schischko, A., Ren, H., Kaplaneris, N. & Ackermann, L. Bioorthogonal diversification of peptides through selective ruthenium(II)-catalyzed C–H activation. Angew. Chem. Int. Ed.56, 1576–1580 (2017). Google Scholar
- Schischko, A. et al. Late-stage peptide C–H alkylation for bioorthogonal C–H activation featuring solid phase peptide synthesis. Nat. Commun.10, 3553 (2019). ADSGoogle Scholar
- Ruan, Z., Sauermann, N., Manoni, E. & Ackermann, L. Manganese-catalyzed C–H alkynylation: expedient peptide synthesis and modification. Angew. Chem. Int. Ed.56, 3172–3176 (2017). Google Scholar
- Wang, W., Subramanian, P., Martinazzoli, O., Wu, J. & Ackermann, L. Glycopeptides by linch-pin C–H activations for peptide–carbohydrate conjugation by manganese(I)-catalysis. Chem. Eur. J.25, 10585–10589 (2019). Google Scholar
- Lorion, M. M., Kaplaneris, N., Son, J., Kuniyil, R. & Ackermann, L. Late-stage peptide diversification through cobalt-catalyzed C–H activation: sequential multicatalysis for stapled peptides. Angew. Chem. Int. Ed.58, 1684–1688 (2019). Google Scholar
- Peng, J., Li, C., Khamrakulov, M., Wang, J. & Liu, H. Rhodium(III)-catalyzed C–H alkenylation: access to maleimide-decorated tryptophan and tryptophan-containing peptides. Org. Lett.22, 1535–1541 (2020). Google Scholar
- Gong, W., Zhang, G., Liu, T., Giri, R. & Yu, J. Q. Site-selective C(sp 3 )–H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J. Am. Chem. Soc.136, 16940–16946 (2014). Google Scholar
- Tang, J., He, Y., Chen, H., Sheng, W. & Wang, H. Synthesis of bioactive and stabilized cyclic peptides by macrocyclization using C(sp 3 )–H activation. Chem. Sci.8, 4565–4570 (2017). Google Scholar
- Noisier, A. F. M., García, J., Ionuţ, I. A. & Albericio, F. Stapled peptides by late-stage C(sp 3 )–H activation. Angew. Chem. Int. Ed.56, 314–318 (2017). Google Scholar
- Mondal, B., Roy, B. & Kazmaier, U. Stereoselective peptide modifications via β-C(sp 3 )–H arylations. J. Org. Chem.81, 11646–11655 (2016). Google Scholar
- Li, X. et al. Synthesis of cyclophane-braced peptide macrocycles via palladium-catalyzed intramolecular C(sp 3 )–H arylation of N-methyl alanine at C-termini. Org. Lett.22, 0–4 (2020). Google Scholar
- Bauer, M., Wang, W., Lorion, M. M., Dong, C. & Ackermann, L. Internal peptide late-stage diversification: peptide-isosteric triazoles for primary and secondary C(sp 3 )–H activation. Angew. Chem. Int. Ed.57, 203–207 (2018). Google Scholar
- Wang, W., Lorion, M. M., Martinazzoli, O. & Ackermann, L. Bodipy peptide labeling by late-stage C(sp 3 )–H activation. Angew. Chem. Int. Ed.57, 10554–10558 (2018). Google Scholar
- Wu, J., Kaplaneris, N., Ni, S., Kaltenhäuser, F. & Ackermann, L. Late-stage C(sp 2 )–H and C(sp 3 )–H glycosylation of C-aryl/alkyl glycopeptides: mechanistic insights and fluorescence labeling. Chem. Sci.11, 6521–6526 (2020). Google Scholar
- Zhan, B. B., Fan, J., Jin, L. & Shi, B. F. Divergent synthesis of silicon-containing peptides via Pd-catalyzed post-assembly γ-C(sp 3 )–H silylation. ACS Catal.9, 3298–3303 (2019). Google Scholar
- Li, B. et al. Construction of natural-product-like cyclophane-braced peptide macrocycles via sp 3 C–H arylation. J. Am. Chem. Soc.141, 9401–9407 (2019). Google Scholar
- Liu, L., Liu, Y. H. & Shi, B. F. Synthesis of amino acids and peptides with bulky side chains: via ligand-enabled carboxylate-directed γ-C(sp 3 )–H arylation. Chem. Sci.11, 290–294 (2020). Google Scholar
- Weng, Y. et al. Peptide late-stage C(sp 3 )–H arylation by native asparagine assistance without exogenous directing group. Chem. Sci.11, 9290–9295 (2020). Google Scholar
- Pouliot, J.-R., Grenier, F., Blaskovits, J. T., Beaupré, S. & Leclerc, M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev.116, 14225–14274 (2016). Google Scholar
- Nielsen, K. T., Bechgaard, K. & Krebs, F. C. Removal of palladium nanoparticles from polymer materials. Macromolecules38, 658–659 (2005). ADSGoogle Scholar
- Ishikawa, T., Motoyanagi, J. & Minoda, M. Synthesis of brush-shaped π-conjugated polymers based on well-defined thiophene-end-capped poly(vinyl ether)s. Chem. Lett.45, 415–417 (2016). Google Scholar
- Okamoto, K., Housekeeper, J. B., Michael, F. E. & Luscombe, C. K. Thiophene based hyperbranched polymers with tunable branching using direct arylation methods. Polym. Chem.4, 3499–3506 (2013). Google Scholar
- Liu, D.-P. et al. Fluorinated porous organic polymers via direct C–H arylation polycondensation. ACS Macro Lett.2, 522–526 (2013). Google Scholar
- Schipper, D. J. & Fagnou, K. Direct arylation as a synthetic tool for the synthesis of thiophene-based organic electronic materials. Chem. Mater.23, 1594–1600 (2011). Google Scholar
- Gobalasingham, N. S., Noh, S. & Thompson, B. C. Palladium-catalyzed oxidative direct arylation polymerization (Oxi-DArP) of an ester-functionalized thiophene. Polym. Chem.7, 1623–1631 (2016). Google Scholar
- Lu, W., Kuwabara, J. & Kanbara, T. Polycondensation of dibromofluorene analogues with tetrafluorobenzene via direct arylation. Macromolecules44, 1252–1255 (2011). ADSGoogle Scholar
- Bohra, H. & Wang, M. Direct C–H arylation: a ‘Greener’ approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A5, 11550–11571 (2017). Google Scholar
- Gather, M. C., Köhnen, A. & Meerholz, K. White organic light-emitting diodes. Adv. Mater.23, 233–248 (2011). Google Scholar
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater.23, 733–758 (2011). Google Scholar
- Jin, Y. et al. A novel naphtho[1,2-c:5,6-c′]bis([1,2,5]thiadiazole)-based narrow-bandgap π-conjugated polymer with power conversion efficiency over 10%. Adv. Mater.28, 9811–9818 (2016). Google Scholar
- Wakioka, M., Kitano, Y. & Ozawa, F. A highly efficient catalytic system for polycondensation of 2,7-dibromo-9,9-dioctylfluorene and 1,2,4,5-tetrafluorobenzene via direct arylation. Macromolecules46, 370–374 (2013). ADSGoogle Scholar
- Pankow, R. M., Ye, L. & Thompson, B. C. Copper catalyzed synthesis of conjugated copolymers using direct arylation polymerization. Polym. Chem.9, 4120–4124 (2018). Google Scholar
- Kuwabara, J. et al. Synthesis of conjugated polymers via direct C–H/C–Cl coupling reactions using a Pd/Cu binary catalytic system. Polym. Chem.10, 2298–2304 (2019). Google Scholar
- Tsuchiya, K. & Ogino, K. Catalytic oxidative polymerization of thiophene derivatives. Polym. J.45, 281–286 (2013). Google Scholar
- Huang, Q. et al. Cu-catalysed oxidative C–H/C–H coupling polymerisation of benzodiimidazoles: an efficient approach to regioregular polybenzodiimidazoles for blue-emitting materials. Chem. Commun.50, 13739–13741 (2014). Google Scholar
- Della, Ca’, N., Fontana, M., Motti, E. & Catellani, M. Pd/Norbornene: a winning combination for selective aromatic functionalization via C–H bond activation. Acc. Chem. Res.49, 1389–1400 (2016). Google Scholar
- Liu, S., Jin, Z., Teo, Y. C. & Xia, Y. Efficient synthesis of rigid ladder polymers via palladium catalyzed annulation. J. Am. Chem. Soc.136, 17434–17437 (2014). Google Scholar
- Lai, H. W. H. et al. Tuning the molecular weights, chain packing, and gas-transport properties of CANAL ladder polymers by short alkyl substitutions. Macromolecules52, 6294–6302 (2019). ADSGoogle Scholar
- Ma, X. et al. Facile synthesis and study of microporous catalytic arene–norbornene annulation — Tröger’s base ladder polymers for membrane air separation. ACS Macro Lett.9, 680–685 (2020). Google Scholar
- Yang, Y., Nishiura, M., Wang, H. & Hou, Z. Metal-catalyzed CH activation for polymer synthesis and functionalization. Coord. Chem. Rev.376, 506–532 (2018). Google Scholar
- Ringelberg, S. N., Meetsma, A., Hessen, B. & Teuben, J. H. Thiophene C–H activation as a chain-transfer mechanism in ethylene polymerization: catalytic formation of thienyl-capped polyethylene. J. Am. Chem. Soc.121, 6082–6083 (1999). Google Scholar
- Yamamoto, A., Nishiura, M., Oyamada, J., Koshino, H. & Hou, Z. Scandium-catalyzed syndiospecific chain-transfer polymerization of styrene using anisoles as a chain transfer agent. Macromolecules49, 2458–2466 (2016). ADSGoogle Scholar
- Kaneko, H., Nagae, H., Tsurugi, H. & Mashima, K. End-functionalized polymerization of 2-vinylpyridine through initial C–H bond activation of N-heteroaromatics and internal alkynes by yttrium ene–diamido complexes. J. Am. Chem. Soc.133, 19626–19629 (2011). Google Scholar
- Schaffer, A., Kränzlein, M. & Rieger, B. Precise synthesis of poly(dimethylsiloxane) copolymers through C–H bond-activated macroinitiators via yttrium-mediated group transfer polymerization and ring-opening polymerization. Macromolecules53, 8382–8392 (2020). ADSGoogle Scholar
- Perry, M. R. et al. Catalytic synthesis of secondary amine-containing polymers: variable hydrogen bonding for tunable rheological properties. Macromolecules49, 4423–4430 (2016). ADSGoogle Scholar
- Kuanr, N. et al. Dynamic cross-linking of catalytically synthesized poly(aminonorbornenes). Macromolecules53, 2649–2661 (2020). ADSGoogle Scholar
- Guo, H., Tapsak, M. A. & Weber, W. P. Ruthenium-catalyzed regioselective step-growth copolymerization of p-(dialkylamino)acetophenones and α,ω-dienes. Macromolecules28, 4714–4718 (1995). ADSGoogle Scholar
- Shin, J. et al. Controlled functionalization of crystalline polystyrenes via activation of aromatic C–H bonds. Macromolecules40, 8600–8608 (2007). ADSGoogle Scholar
- Kondo, Y. et al. Rhodium-catalyzed, regiospecific functionalization of polyolefins in the melt. J. Am. Chem. Soc.124, 1164–1165 (2002). Google Scholar
- Gupta, S. K. & Weber, W. P. Ruthenium-catalyzed chemical modification of poly(vinylmethylsiloxane) with 9-acetylphenanthrene. Macromolecules35, 3369–3373 (2002). ADSGoogle Scholar
- Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev.45, 546–576 (2016). This review outlines the strategies for LSF very well. Google Scholar
- Börgel, J. & Ritter, T. Late-stage functionalization. Chem6, 1877–1887 (2020). Google Scholar
- Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem.10, 383–394 (2018). Google Scholar
- Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev.45, 2900–2936 (2016). Google Scholar
- Dai, H.-X., Stepan, A. F., Plummer, M. S., Zhang, Y.-H. & Yu, J.-Q. Divergent C–H functionalizations directed by sulfonamide pharmacophores: late-stage diversification as a tool for drug discovery. J. Am. Chem. Soc.133, 7222–7228 (2011). This article presents a divergent approach to SAR exploration by C–H functionalization. Google Scholar
- Tomberg, A. et al. Relative strength of common directing groups in palladium-catalyzed aromatic C–H activation. iScience20, 373–391 (2019). ADSGoogle Scholar
- Li, J., De Sarkar, S. & Ackermann, L. Meta- and para-selective C–H functionalization by C–H activation. Top. Organomet. Chem.55, 217–257 (2016). Google Scholar
- Friis, S. D., Johansson, M. J. & Ackermann, L. Cobalt-catalysed C–H methylation for late-stage drug diversification. Nat. Chem.12, 511–519 (2020). This study achieves the late-stage diversification of various natural products and drugs by C–H methylation under sustainable cobalt catalysis. Google Scholar
- Wencel-Delord, J. Decorating and diversifying drugs. Nat. Chem.12, 505–506 (2020). Google Scholar
- Rodrigalvarez, J. et al. Catalytic C(sp 3 )–H bond activation in tertiary alkylamines. Nat. Chem.12, 76–81 (2020). This publication achieves challenging C(sp3)–H activations in tertiary alkylamines employing a palladium/mono-protected amino acid catalyst. Google Scholar
- Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev.110, 890–931 (2010). Google Scholar
- Larsen, M. A. & Hartwig, J. F. Iridium-catalyzed C–H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism. J. Am. Chem. Soc.136, 4287–4299 (2014). This article fully investigates the scope of iridium-catalysed C–H borylation of heteroarenes, including guidelines for the regiochemical outcome of these processes. Google Scholar
- Ahneman, D. T., Estrada, J. G., Lin, S., Dreher, S. D. & Doyle, A. G. Predicting reaction performance in C–N cross-coupling using machine learning. Science360, 186–190 (2018). ADSGoogle Scholar
- Loup, J., Dhawa, U., Pesciaioli, F., Wencel-Delord, J. & Ackermann, L. Enantioselective C–H activation with earth-abundant 3d transition metals. Angew. Chem. Int. Ed.58, 12803–12818 (2019). This review summarizes recent developments in the challenging area of asymmetric C–H activation under environmentally benign 3dtransition metal catalysis. Google Scholar
- Fan, Z. et al. Rational development of remote C–H functionalization of biphenyl: experimental and computational studies. Angew. Chem. Int. Ed.59, 4770–4777 (2020). This combined experimental and computational study discloses efficient and selective nitrile-directedmeta-C–H activations, proceeding through a hetero-bimetallic silver–palladium intermediate. Google Scholar
- Yu, Q., Hu, L., Wang, Y., Zheng, S. & Huang, J. Directed meta-selective bromination of arenes with ruthenium catalysts. Angew. Chem. Int. Ed.54, 15284–15288 (2015). Google Scholar
- Shen, P.-X., Wang, X.-C., Wang, P., Zhu, R.-Y. & Yu, J.-Q. Ligand-enabled meta-C–H alkylation and arylation using a modified norbornene. J. Am. Chem. Soc.137, 11574–11577 (2015). Google Scholar
- Wang, X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature519, 334–338 (2015). ADSGoogle Scholar
- Meng, G. et al. Achieving site-selectivity for C–H activation processes based on distance and geometry: a carpenter’s approach. J. Am. Chem. Soc.142, 10571–10591 (2020). Google Scholar
- Funes-Ardoiz, I. & Maseras, F. Oxidative coupling mechanisms: current state of understanding. ACS Catal.8, 1161–1172 (2018). Google Scholar
- Ano, Y. & Chatani, N. ortho-Directed C–H alkylation of substituted benzenes. Org. React.100, 622–670 (2019). Google Scholar
- Guillemard, L. & Wencel-Delord, J. When metal-catalyzed C–H functionalization meets visible-light photocatalysis. Beilstein J. Org. Chem.16, 1754–1804 (2020). Google Scholar
- Capaldo, L., Quadri, L. L. & Ravelli, D. Merging photocatalysis with electrochemistry: the dawn of a new alliance in organic synthesis. Angew. Chem. Int. Ed.58, 17508–17510 (2019). Google Scholar
- Samanta, R. C., Meyer, T. H., Siewert, I. & Ackermann, L. Renewable resources for sustainable metallaelectro-catalyzed C–H activation. Chem. Sci.11, 8657–8670 (2020). Google Scholar
- Gandeepan, P., Finger, L. H., Meyer, T. H. & Ackermann, L. 3d metallaelectrocatalysis for resource economical syntheses. Chem. Soc. Rev.49, 4254–4272 (2020). Google Scholar
- Jorner, K., Tomberg, A., Bauer, C., Sköld, C. & Norrby, P.-O. Organic reactivity from mechanism to machine learning. Nat. Rev. Chem.5, 240–255 (2021). Google Scholar
- Struble, T. J., Coley, C. W. & Jensen, K. F. Multitask prediction of site selectivity in aromatic C–H functionalization reactions. React. Chem. Eng.5, 896–902 (2020). Google Scholar
- Dwivedi, V., Kalsi, D. & Sundararaju, B. Electrochemical-/photoredox aspects of transition metal-catalyzed directed C–H bond activation. ChemCatChem11, 5160–5187 (2019). Google Scholar
- De Abreu, M., Belmont, P. & Brachet, E. Synergistic photoredox/transition-metal catalysis for carbon–carbon bond formation reactions. Eur. J. Org. Chem.2020, 1327–1378 (2020). Google Scholar
- Fabry, D. C. & Rueping, M. Merging visible light photoredox catalysis with metal catalyzed C–H activations: on the role of oxygen and superoxide ions as oxidants. Acc. Chem. Res.49, 1969–1979 (2016). Google Scholar
- Zoller, J., Fabry, D. C., Ronge, M. A. & Rueping, M. Synthesis of indoles using visible light: photoredox catalysis for palladium-catalyzed C–H activation. Angew. Chem. Int. Ed.53, 13264–13268 (2014). Google Scholar
- Fabry, D. C., Zoller, J., Raja, S. & Rueping, M. Combining rhodium and photoredox catalysis for C–H functionalizations of arenes: oxidative Heck reactions with visible light. Angew. Chem. Int. Ed.53, 10228–10231 (2014). Google Scholar
- Fabry, D. C., Ronge, M. A., Zoller, J. & Rueping, M. C–H functionalization of phenols using combined ruthenium and photoredox catalysis: in situ generation of the oxidant. Angew. Chem. Int. Ed.54, 2801–2805 (2015). Google Scholar
- Kalyani, D., McMurtrey, K. B., Neufeldt, S. R. & Sanford, M. S. Room-temperature C–H arylation: merger of Pd-catalyzed C–H functionalization and visible-light photocatalysis. J. Am. Chem. Soc.133, 18566–18569 (2011). Google Scholar
- Sahoo, M. K., Midya, S. P., Landge, V. G. & Balaraman, E. A unified strategy for silver-, base-, and oxidant-free direct arylation of C–H bonds. Green Chem.19, 2111–2117 (2017). Google Scholar
- Jiang, J., Zhang, W.-M., Dai, J.-J., Xu, J. & Xu, H.-J. Visible-light-promoted C–H arylation by merging palladium catalysis with organic photoredox catalysis. J. Org. Chem.82, 3622–3630 (2017). Google Scholar
- Zhou, C., Li, P., Zhu, X. & Wang, L. Merging photoredox with palladium catalysis: decarboxylative ortho-acylation of acetanilides with α-oxocarboxylic acids under mild reaction conditions. Org. Lett.17, 6198–6201 (2015). Google Scholar
- Sharma, U. K., Gemoets, H. P. L., Schröder, F., Noël, T. & & Van der Eycken, E. V. Merger of visible-light photoredox catalysis and C–H activation for the room-temperature C-2 acylation of indoles in batch and flow. ACS Catal.7, 3818–3823 (2017). Google Scholar
- Gandeepan, P., Koeller, J., Korvorapun, K., Mohr, J. & Ackermann, L. Visible-light for ruthenium-catalyzed meta-C–H alkylation at room temperature. Angew. Chem. Int. Ed.58, 9820–9825 (2019). Google Scholar
- Greaney, M. & Sagadevan, A. Meta-selective C–H activation of arenes at room temperature using visible light: dual-function ruthenium catalysis. Angew. Chem. Int. Ed.58, 9826–9830 (2019). Google Scholar
- Thongpaen, J. et al. Visible light induced rhodium(I)-catalyzed C–H borylation. Angew. Chem. Int. Ed.58, 15244–15248 (2019). Google Scholar
- Gauchot, V., Sutherland, D. R. & Lee, A. L. Dual gold and photoredox catalysed C–H activation of arenes for aryl–aryl cross couplings. Chem. Sci.8, 2885–2889 (2017). Google Scholar
- Liang, Y.-F., Steinbock, R., Yang, L. & Ackermann, L. Continuous visible-light photoflow approach for a manganese-catalyzed (het)arene C–H arylation. Angew. Chem. Int. Ed.57, 10625–10629 (2018). Google Scholar
- Yang, F., Koeller, J. & Ackermann, L. Photoinduced copper-catalyzed C–H arylation at room temperature. Angew. Chem. Int. Ed.55, 4759–4762 (2016). Google Scholar
- Tlahuext-Aca, A., Hopkinson, M. N., Sahoo, B. & Glorius, F. Dual gold/photoredox-catalyzed C(sp)–H arylation of terminal alkynes with diazonium salts. Chem. Sci.7, 89–93 (2016). Google Scholar
- Ackermann, L. Metalla-electrocatalyzed C–H activation by earth-abundant 3d metals and beyond. Acc. Chem. Res.53, 84–104 (2020). Google Scholar
- Ma, C., Fang, P. & Mei, T.-S. Recent advances in C–H functionalization using electrochemical transition metal catalysis. ACS Catal.8, 7179–7189 (2018). Google Scholar
- Kärkäs, M. D. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev.47, 5786–5865 (2018). Google Scholar
- Kathiravan, S., Suriyanarayanan, S. & Nicholls, I. A. Electrooxidative amination of sp 2 C–H bonds: coupling of amines with aryl amides via copper catalysis. Org. Lett.21, 1968–1972 (2019). Google Scholar
- Zhang, S.-K., Samanta, R. C., Sauermann, N. & Ackermann, L. Nickel-catalyzed electrooxidative C–H amination: support for nickel(IV). Chem. Eur. J.24, 19166–19170 (2018). Google Scholar
- Tang, S., Wang, D., Liu, Y., Zeng, L. & Lei, A. Cobalt-catalyzed electrooxidative C–H/N–H [4 + 2] annulation with ethylene or ethyne. Nat. Commun.9, 798 (2018). ADSGoogle Scholar
- Qiu, Y., Tian, C., Massignan, L., Rogge, T. & Ackermann, L. Electrooxidative ruthenium-catalyzed C–H/O–H annulation by weak O-coordination. Angew. Chem. Int. Ed.57, 5818–5822 (2018). Google Scholar
- Ma, C. et al. Palladium-catalyzed C–H activation/C–C cross-coupling reactions via electrochemistry. Chem. Commun.53, 12189–12192 (2017). Google Scholar
- Kakiuchi, F. et al. Palladium-catalyzed aromatic C–H halogenation with hydrogen halides by means of electrochemical oxidation. J. Am. Chem. Soc.131, 11310–11311 (2009). Google Scholar
- Amatore, C., Cammoun, C. & Jutand, A. Electrochemical recycling of benzoquinone in the Pd/benzoquinone-catalyzed Heck-type reactions from arenes. Adv. Synth. Catal.349, 292–296 (2007). Google Scholar
- Samanta, R. C., Struwe, J. & Ackermann, L. Nickela-electrocatalyzed mild C–H alkylations at room temperature. Angew. Chem. Int. Ed.59, 14154–14159 (2020). Google Scholar
- Qiu, Y., Scheremetjew, A., Finger, L. H. & Ackermann, L. Electrophotocatalytic undirected C–H trifluoromethylations of (het)arenes. Chem. Eur. J.26, 3241–3246 (2020). Google Scholar
- Wang, F. & Stahl, S. S. Merging photochemistry with electrochemistry: functional-group tolerant electrochemical amination of C(sp 3 )–H bonds. Angew. Chem. Int. Ed.58, 6385–6390 (2019). Google Scholar
- Kong, W.-J. et al. Flow rhodaelectro-catalyzed alkyne annulations by versatile C–H activation: mechanistic support for rhodium(III/IV). J. Am. Chem. Soc.141, 17198–17206 (2019). Google Scholar
- Noël, T., Cao, Y. & Laudadio, G. The fundamentals behind the use of flow reactors in electrochemistry. Acc. Chem. Res.52, 2858–2869 (2019). Google Scholar
- Meyer, T. H., Choi, I., Tian, C. & Ackermann, L. Powering the future: how can electrochemistry make a difference in organic synthesis? Chem6, 2484–2496 (2020). Google Scholar
- Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem.5, 369–375 (2013). Google Scholar
- Mehta, A., Saha, B., Koohang, A. A. & Chorghade, M. S. Arene ruthenium catalyst MCAT-53 for the synthesis of heterobiaryl compounds in water through aromatic C–H bond activation. Org. Process. Res. Dev.22, 1119–1130 (2018). Google Scholar
- Ackermann, L. Robust ruthenium(II)-catalyzed C–H arylations: carboxylate assistance for the efficient synthesis of angiotensin-II-receptor blockers. Org. Process. Res. Dev.19, 260–269 (2015). Google Scholar
- Park, Y., Jee, S., Kim, J. G. & Chang, S. Study of sustainability and scalability in the Cp*Rh(III)-catalyzed direct C–H amidation with 1,4,2-dioxazol-5-ones. Org. Process. Res. Dev.19, 1024–1029 (2015). Google Scholar
- Pitzer, L., Schafers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed.58, 8572–8576 (2019). Google Scholar
- Mennen, S. M. et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process Res. Dev.23, 1213–1242 (2019). Google Scholar
- Grimme, S. & Schreiner, P. R. Computational chemistry: the fate of current methods and future challenges. Angew. Chem. Int. Ed.57, 4170–4176 (2018). Google Scholar
- Gandeepan, P., Kaplaneris, N., Santoro, S., Vaccaro, L. & Ackermann, L. Biomass-derived solvents for sustainable transition metal-catalyzed C–H activation. ACS Sustain. Chem. Eng.7, 8023–8040 (2019). Google Scholar
- Santoro, S., Kozhushkov, S. I., Ackermann, L. & Vaccaro, L. Heterogeneous catalytic approaches in C–H activation reactions. Green Chem.18, 3471–3493 (2016). Google Scholar
- Strieth-Kalthoff, F., Sandfort, F., Segler, M. H. S. & Glorius, F. Machine learning the ropes: principles, applications and directions in synthetic chemistry. Chem. Soc. Rev.49, 6154–6168 (2020). Google Scholar
- Nugent, W. A., Ovenall, D. W. & Holmes, S. J. Catalytic C–H activation in early transition-metal dialkylamides and alkoxides. Organometallics2, 161–162 (1983). Google Scholar
- Gilmour, D. J., Lauzon, J. M. P., Clot, E. & Schafer, L. L. Ta-catalyzed hydroaminoalkylation of alkenes: insights into ligand-modified reactivity using DFT. Organometallics37, 4387–4394 (2018). Google Scholar
- Maspero, F. & Clerici, M. G. Catalytic C-alkylation of secondary amines with alkenes. Synthesis1980, 305–306 (1980). Google Scholar
- Herzon, S. B. & Hartwig, J. F. Direct, catalytic hydroaminoalkylation of unactivated olefins with N-alkyl arylamines. J. Am. Chem. Soc.129, 6690–6691 (2007). Google Scholar
- Prochnow, I., Zark, P., Müller, T. & Doye, S. The mechanism of the titanium-catalyzed hydroaminoalkylation of alkenes. Angew. Chem. Int. Ed.50, 6401–6405 (2011). Google Scholar
- Bexrud, J. A., Eisenberger, P., Leitch, D. C., Payne, P. R. & Schafer, L. L. Selective C–H activation α to primary amines. Bridging metallaaziridines for catalytic, intramolecular α-alkylation. J. Am. Chem. Soc.131, 2116–2118 (2009). Google Scholar
- Chong, E. & Schafer, L. L. 2-Pyridonate titanium complexes for chemoselectivity. accessing intramolecular hydroaminoalkylation over hydroamination. Org. Lett.15, 6002–6005 (2013). Google Scholar
- Rosien, M., Töben, I., Schmidtmann, M., Beckhaus, R. & Doye, S. Titanium-catalyzed hydroaminoalkylation of ethylene. Chem. Eur. J.26, 2138–2142 (2020). Google Scholar
- Daneshmand, P. et al. Cyclic ureate tantalum catalyst for preferential hydroaminoalkylation with aliphatic amines: mechanistic insights into substrate controlled reactivity. J. Am. Chem. Soc.142, 15740–15750 (2020). Google Scholar
- Payne, P. R., Garcia, P., Eisenberger, P., Yim, J. C. H. & Schafer, L. L. Tantalum catalyzed hydroaminoalkylation for the synthesis of α- and β-substituted N-heterocycles. Org. Lett.15, 2182–2185 (2013). Google Scholar
- Doerfler, J., Preuss, T., Schischko, A., Schmidtmann, M. & Doye, S. A 2,6-bis(phenylamino)pyridinato titanium catalyst for the highly regioselective hydroaminoalkylation of styrenes and 1,3-butadienes. Angew. Chem. Int. Ed.53, 7918–7922 (2014). Google Scholar
- Bielefeld, J., Mannhaupt, S., Schmidtmann, M. & Doye, S. Hydroaminoalkylation of allenes. Synlett30, 967–971 (2019). Google Scholar
- Liu, F., Luo, G., Hou, Z. & Luo, Y. Mechanistic insights into scandium-catalyzed hydroaminoalkylation of olefins with amines: origin of regioselectivity and charge-based prediction model. Organometallics36, 1557–1565 (2017). Google Scholar
- Reznichenko, A. L. & Hultzsch, K. C. The mechanism of hydroaminoalkylation catalyzed by group 5 metal binaphtholate complexes. J. Am. Chem. Soc.134, 3300–3311 (2012). Google Scholar
- Zi, G., Zhang, F. & Song, H. Highly enantioselective hydroaminoalkylation of secondary amines catalyzed by group 5 metal amides with chiral biarylamidate ligands. Chem. Commun.46, 6296–6298 (2010). Google Scholar
- Braun, C., Nieger, M., Bräse, S. & Schafer, L. L. Planar-chiral [2.2]paracyclophane-based pyridonates as ligands for tantalum-catalyzed hydroaminoalkylation. ChemCatChem11, 5264–5268 (2019). Google Scholar
- Nako, A. E., Oyamada, J., Nishiura, M. & Hou, Z. Scandium-catalysed intermolecular hydroaminoalkylation of olefins with aliphatic tertiary amines. Chem. Sci.7, 6429–6434 (2016). Google Scholar
- Su, J., Zhou, Y. & Xu, X. Hydroaminoalkylation of sterically hindered alkenes with N,N-dimethyl anilines using a scandium catalyst. Org. Biomol. Chem.17, 2013–2019 (2019). Google Scholar
Acknowledgements
Generous support by the Deutsche Forschungsgemeinschaft (DFG) (SPP 1807 and Gottfried–Wilhelm–Leibniz prize to L.A.) and the Onassis Foundation (fellowship to N.K.) is gratefully acknowledged. The research leading to these results has received funding from the NMBP‐01–2016 Program of the European Union’s Horizon 2020 Framework Program H2020/2014–2020 under Grant Agreement No. 720996. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie Grant Agreement No. 860762. D.G.M. acknowledges the National Science Foundation (NSF) grant under the CCI Center for Selective C–H Functionalization (CHE-1700982). Z.E.W. and M.A.B. thank the Maurice Wilkins Centre for Molecular Biodiscovery for financial support. J.W.-D. thanks the CNRS (Centre National de la Recherche Scientifique), the Ministere de l’Education Nationale et de la Recherche France and the ANR-DFG programme, grant number Projet ANR-17-CE07-0049-01. This work was supported by a Grant in Aid for Specially Promoted Research by MEXT (No. 17H06091). M.J.J. thanks the Swedish Foundation for Strategic Environmental Research (Mistra; project Mistra SafeChem).
Author information
Authors and Affiliations
- Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Göttingen, Germany Torben Rogge, Nikolaos Kaplaneris & Lutz Ackermann
- Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka, Japan Naoto Chatani
- Center for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, South Korea Jinwoo Kim & Sukbok Chang
- Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, South Korea Jinwoo Kim & Sukbok Chang
- Organometallic Synthesis and Catalysis Lab, Chemical Engineering Division, CSIR–National Chemical Laboratory (CSIR–NCL), Pune, India Benudhar Punji
- Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India Benudhar Punji
- Department of Chemistry, University of British Columbia, Vancouver, British Columbia, Canada Laurel L. Schafer
- Cherry L. Emerson Center, Department of Chemistry, Emory University, Atlanta, GA, USA Djamaladdin G. Musaev
- Laboratoire d’Innovation Moléculaire et Applications (UMR CNRS7042), Université de Strasbourg/Université de Haute Alsace, ECPM, Strasbourg, France Joanna Wencel-Delord
- Department of Chemistry, University of California, Berkeley, CA, USA Charis A. Roberts & Richmond Sarpong
- School of Chemical Sciences, University of Auckland, Auckland, New Zealand Zoe E. Wilson & Margaret A. Brimble
- School of Biological Sciences, University of Auckland, Auckland, New Zealand Zoe E. Wilson & Margaret A. Brimble
- Maurice Wilkins Centre for Molecular Biodiscovery, Auckland, New Zealand Zoe E. Wilson & Margaret A. Brimble
- Medicinal Chemistry, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), Biopharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden Magnus J. Johansson
- Department of Organic Chemistry, Stockholm University, Stockholm, Sweden Magnus J. Johansson
- Torben Rogge
















